These stories refer to incidents where toughened glass windows shatter without warning. Although it is only recently that this so-called "spontaneous" failure of toughened glass has come to public attention, it has been known about since 1960. These failures are due to the presence of nickel sulphide inclusions. In fact, nickel sulphide inclusions in glass are quite rare. In a typical glass batch there will be one 5 µg inclusion per tonne of glass (an average concentration of 5 parts in 1012).
Though rare, the nickel sulphide inclusions are very troublesome and potentially dangerous when present in toughened glass. The reason for all the trouble is a delayed phase transformation in nickel sulphide. Nickel sulphide crystals have a high temperature and a low temperature form. The dense crystal form at high temperature swells on cooling to make a less dense crystal form at low temperatures.
In ordinary annealed glass nickel sulphide inclusions do not cause problems because the transformation occurs as the glass is cooled slowly during manufacture. However, the transformation is sluggish and when glass is rapidly cooled as part of the toughening process, the nickel sulphide remains trapped in its high temperature form until some years later when its transformation breaks the glass.

A characteristic "spontaneous" fracture pattern in a toughened glass window. The nickel sulphide inclusion is found at the initiation of failure (at the centre of the butterfly pattern, arrowed).
The manufacture of toughened glass and generation of unstable nickel sulphide
Flat glass is toughened in an oven that resembles a giant toaster. The glass is transported on rollers and is rolled back and forth inside the oven and heated to a temperature of between 600 and 700 °C until it becomes soft. The glass in its softened state is rolled out of the oven into an air shower where both sides of the glass are cooled rapidly. Glass has low thermal conductivity so that the inside of the glass remains hot and soft while the two outer surfaces of the glass cool, solidify and then contract due to thermal contraction (thermal expansion in reverse).
After this the inside gradually cools, solidifies and contracts. Because the outer surfaces are already cold when the inner region begins to solidify, contraction in the inner region squeezes the outer surfaces. In the finished glass the regions near the outer surfaces experience high compressive forces which are balanced by high tensile forces generated at the inner region. The toughening process produces a safety glass that is also very strong.


Nickel sulphide inclusions found inside intact glass. There is significant cracking in the glass adjacent to the inclusion, but at the time of photography these cracks were sub-critical.
Nickel sulphide is in its high temperature form at above 380 °C and should revert to the low temperature form during cooling to room temperature, but in toughened glass does not do so because the transformation is sluggish and because of the rapid cooling rate required by the toughening process.
Swain found that the high to low temperature transformation results in a 4% expansion of nickel sulphide, so that inclusions larger than 60 µm in diameter can generate potentially dangerous cracks in the surrounding glass. On the positive side, external and internal stresses impart valuable properties to toughened glass.
The compressive stress at the surfaces closes up surface cracks and increases the glass strength by a factor of 3 to 5 times. The internal tensile stress ensures that, if the glass should be broken, then stress release causes the glass to shatter safely into small blocks (fracture dice).
However, if a nickel sulphide inclusion is present in the tensile zone there is a down side and the internal tensile stress becomes an "Achilles heel" for toughened glass. The problem is that swelling of the nickel sulphide inclusions does generate cracks in the glass and any small crack in the tensile zone will cause catastrophic failure.
The sluggish property of the transformation results in a delay between toughening (which generates the unstable inclusion) and glass failure. The rates of failure are hard to predict since both rate of failure and length of time delay vary from location to location. In some installations all failures occur within 5 years, but in many cases failures continue for 10 years or more after installation.


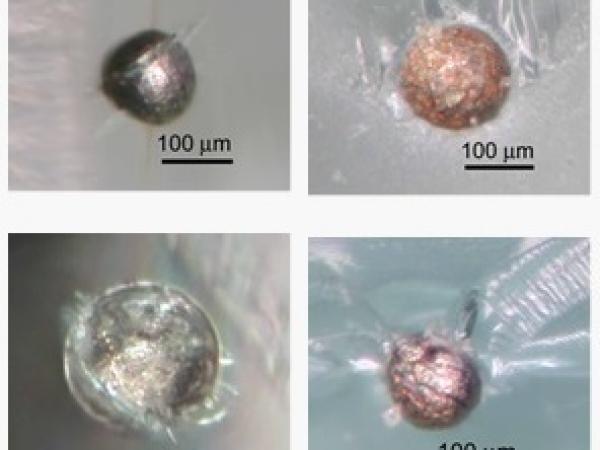
Nickel sulphide inclusions found on fracture surfaces of glass that failed by "spontaneous fracture".
Towards fixing the problem: characterisation, testing and detection
More than 45 years has elapsed since the cause of spontaneous fracture in toughened glass was first established. Since then there have been a number of attempts to eliminate the problem. Three different types of approach have been adopted; which are: characterisation, destructive tests, and detection.


Scanning electron microscopy images of nickel sulphide inclusions found on a fracture surface. The inclusion on the left is quite large (400 µm in diameter), the inclusion on the right is relatively small (85 µm).
Characterisation is a study of a material; what it looks like, what is made of, what kinds of impurities and trace elements does it contain, and how is it situated in relation to its surrounds. By characterisation of the nickel sulphide and other impurities in the glass we can hope to find out something about how the nickel sulphide came to be there and perhaps figure out how to remove it.
We know that sulphur is added as a component in glass manufacture but the sources of nickel are unknown. Characterisation, therefore, is focussed on identification of sources of nickel.
There are three possible sources of nickel; the raw materials, the materials used in storage and handling of raw materials, and contamination coming into the glass melt via fire bricks and the burners. As a result of some early characterisation work it was recognised that fuel oil contains traces of nickel and because of this glass processing tanks which originally used fuel oil burners have been converted to natural gas.
The conversion to natural gas and modifications in handling of raw materials has improved the situation with nickel sulphide but has not eliminated the problem. There have been some recent discoveries made in relation to sources of nickel and these will be discussed further below.
A destructive test called "heat soaking" has been developed for detection and removal of glass which contains nickel sulphide inclusions. In a heat-soak test the toughened glass is stacked inside a hot box and reheated to 290 °C for a few hours. The heat-soak process induces the inclusions to transform to their low temperature form which causes the problem windows to fail.
The advantage in using this process in that the heat-soak removes more than 95% of problem windows and does not significantly reduce the temper of the glass. The disadvantage of the process is that it adds extra cost because of the need for extra processing, and because it is destructive.
All shaping, hole-drilling and finishing of the glass must be done before toughening, so the loss of windows in a destructive test represents a loss of time spent in preparing the glass. In addition, because the sheets are stacked close together on the hot box, the failure of one sheet of glass will often damage adjacent glass as well.

Scanning electron microscopy image of a polished cross-section of a nickel sulphide inclusion. The inclusion is not single phase but rather is a multi-phase assembly of Ni9S8 and Ni1-xS.


Energy dispersive X-ray spectra generated in a scanning electron microscope. A spectrum from dark areas (above) is sulphur rich as compared with a spectrum from the bright areas (below).
Detection of nickel sulphide inclusions
The last of the three approaches is detection. Detection has benefits that a destructive test does not. Although the heat-soak test is effective it has two drawbacks, firstly it is wasteful and secondly it cannot be easily applied in existing installations where there are known problems with nickel sulphide. However, detection of inclusions is quite challenging because they are so small.
When spontaneous fracture does occur the majority of inclusions found at the initiation of failure are between 100 and 200 µm in diameter. And in some cases inclusions as small as 70 µm have been found. The small size of the defect in comparison to the large size of windows means that the detection method will require the processing of a large amount of data in order to reliably detect inclusions.
For example, to digitally register the position of a 70 µm inclusion a pixel size of 35 µm is required. A digital image of 3 m2 window at 35 µm resolution requires 3Gbytes of data storage. Irrespective of the type or sensitivity of a detection technique, the challenge is to be able to process large amounts of data in the shortest possible time.
In spite of the inherent challenges a detection method has been successfully developed and applied. A photographic method developed in a partnership between the University of Queensland and Resolve Engineering was used to examine 4194 individual panes of external vision glass (a total of 14,753 m2 of glass) in a Brisbane building in 1995/96. Using this process, which is known as the photoglass process, 291 nickel sulphide inclusions were found in 281 windows (10 sheets of glass contained 2 nickel sulphide inclusions).
The detection process, which is described in a patent by the author "detection of defects in glass" (Australian patent 732132, US patent 6236734) is done in a three stages; which are: photography, film examination, and glass inspection. In the first stage the windows were photographed using wide-format (120 mm roll film) cameras. Since lens tilt was required of the camera and fine definition required for the film, both camera and film were quite specialised.
For this process we needed to develop a rig strong and stable enough for vibration-free photography which could be performed on the side of the building from a BMU (building maintenance unit). The rig also contained a mechanism for recording height and window number so that it was possible to relate position in film to position in glass.
In the second stage the films were examined in modified microfiche readers. In this way the defects could be viewed by film examiners at 9 times magnification and the film examination done in a more comfortable environment (as compared with direct viewing of the glass). The microfiche readers were modified so as to be able to consistently scan the film one frame at time and to be able to relate scan position to position on the film.
When the film examiners found a characteristic pattern (known as a "dot pair") they entered its position-in-film into a database. By processing information in this database it was possible to relate position-in film to the actual position-in-glass. The "dot pair") images are generated by inclusions in glass.
The spacing of dots in the "dot pairs") is used to measure the depth in glass of the inclusions and to decide whether or not they are in the tension zone. Only inclusions near the centre of the glass (in the tension zone) are dangerous, whereas inclusions near to the outside surfaces cannot break the glass.
In the third stage window examiners were provided with lists of the suspect inclusions found by film examiners. Using the position in glass as provided by the database, the window examiners were able to locate the small inclusions and examine them closely using a 10x lupe eyepiece.
At 10x magnification nickel sulphides are easily identified because of their golden-brown colour and rough surface texture. For the Brisbane building the film examiners identified 53,594 inclusions within the body of the glass and from these the window examiners found 291 nickel sulphide inclusions.
Potential for the development of new detection methods
The photoglass method is a wonderful method for detection but it is limited by the fact that this detection method requires intensive application of human observation. As a result the method is very labour intensive. It would make sense to develop an automated and computer driven method of detection.
During the application of the photoglass method we thoroughly examined all aspects of the detection process and developed very clear specifications for the various detection parameters. We now know exactly what is required for a detection method to be successful.
In 1995/96 the exacting requirements for detection were beyond what could be easily provided by the CCD cameras and computer processors of that time. However, in the intervening 10 years the speed of computer processors has increased by a factor of more than 10 times and also CCD cameras are now much faster and with larger array sizes. Although detection of nickel sulphide inclusions is not simple it is now technically possible to develop a fast and efficient automated detection system. In the first instance it would make sense to develop a system, not to scan all glass, but rather to scan and certify glass that is scheduled for toughening.
The development of a new detection process will require significant resources in the first instance. And of course, as with any successful new development, it will involve risk. Because of nickel sulphide architects and builders have been scared off using toughened glass in recent years. It is a shame not to be using toughened glass since it has properties of clarity, strength and safety which are unmatched by any other material. The time is ripe to develop a new defect detection technique for toughened glass and to repair damage to its reputation.
Where does the nickel come from? Some recent work in characterisation
As a final observation on the value of characterisation I draw attention to some early work and some recent work. Tabuchi had found (in 1974) that there are three different types of nickel sulphide inclusion in glass. Although its relevance was not fully appreciated at the time this finding holds the key to how nickel sulphide is formed and stabilised within the glass melt.
The first type found by Tabuchi is a two phase assembly of nickel metal and Ni3S2 (heazlewoodite), the second type contained the Ni3S2 phase alone, and the third type had a composition close to NiS. The first and second types of nickel sulphide inclusion do not cause spontaneous fracture, but the third type (of composition close to NiS) does cause spontaneous fracture. Later workers have shown that these type 3 inclusions range in composition from Ni7S6 to NiS1.03 and are not strictly single phase but are found to contain the phases Ni7S6, Ni9S8, and Ni1-xS.
On the basis of thermodynamic stability nickel sulphide inclusions should not survive in the glass melt. Although nickel sulphide is somewhat insoluble in glass, the oxidation condition in soda-lime-silica glass favours conversion of nickel sulphide into nickel oxide. Nickel oxide is soluble and once formed will quickly dissolve in the glass melt.
However, in recent work Kasper and Stadelmann have generated nickel sulphide inclusions by adding small particles of stainless steel to the glass melt. This work has revealed the significance of the three types of nickel sulphide inclusion that were found by Tabuchi. Kasper and Stadelmann observed a cascading reaction. In the first stage the least noble metals (chromium and manganese) dissolve into the glass converting the stainless steel to an iron-nickel alloy.
In the second stage the iron is removed leaving nickel metal. In the third stage the nickel metal reacts with sulphur to form Ni3S2. For as long as nickel metal is present the Ni3S2 is retained as a stable phase in the glass melt. Once the nickel metal is all consumed the nickel sulphide inclusion will slowly shrink as it begins to react with the glass to form soluble nickel oxide at its surface.
In addition, once the nickel metal is all consumed the metastable phase NiS (the dangerous phase) is favoured over Ni3S2 and so the inclusion loses nickel and converts from Ni3S2 to NiS. Kasper and Stadelmann showed that nickel sulphide can survive as a metastable phase in glass in the presence of nickel metal, and have demonstrated the necessity for absolute avoidance of contact between nickel alloys and the raw materials used for manufacture of soda-lime-silica glass.
Further Reading
J.C. Barry and S. Ford, "An electron microscopic study of nickel sulphide inclusions in toughened glass", J. Materials Science, 36 (2001) 3721-3730.
Trevor, J Ford, "Spontaneous fracture of glass due to nickel sulphide inclusions " risk management and development of a non destructive testing system", International Conference of Building Envelope Systems & Technology, University of Bath, UK, April 1997.
Andreas Kasper and Herbert Stadelmann, "Chemical behaviour of nickel sulfide in soda-lime-silica glass melts", Glass Sci. Technol. 75 (2002), No. 1, 1-11.
M.V. Swain, "Nickel sulphide inclusions in glass: an example of microcracking induced by a volumetric expanding phase change", J. Materials Science 16 (1981), 151-158
H. Tabuchi, "On the study of sulfide inclusions in plate glasses", Proc. 10th International Congress on Glass, Kyoto, (1974) Vol. 3, 54-58
About the Author
Before starting the science consulting company PicaMS Dr John Barry had worked at Oxford University, Arizona State University and at the University of Queensland. Dr Barry has more than 20 years experience in materials science and in application of electron beam methods. He has 80 papers in peer reviewed journals on topics such as: the relationship between structure and properties of materials, nanoscience, and applications and innovations using electron microscopy techniques.
Dr Barry began research into nickel sulphide in toughened glass in 1991, and while still at the University of Queensland Dr Barry developed the Photoglass method in conjunction with Resolve Engineering. In his present position at PicaMS Dr Barry has competed a number of projects related to problems in glass, including a major project on remediation of toughened glass at a Melbourne racecourse.


















Comments
Thanks for the heads up guys. This website is useful.
Johan
Auto glass repair would work well with toughened glass.
T.W.
https://mesawindshields.com