Architectural Glass Laminating Guide - Part 12
Author: Chris Davis – HB Fuller (Kömmerling Chemische Fabrik GmbH)
1. Background
Liquid interlayers present an alternative thermoset solution to more conventional thermoplastic folio materials for bonding glass to itself or to alternative substrate types. The principle of liquid lamination generally requires the void between the substrates to be permanently or temporarily perimeter sealed before the introduction of the pre-cured liquid polymer. It generally requires only one significant phase change from liquid to a cured polymer through either catalyst or ultra-violet light activated photo initiator mechanisms.
Free flowing liquids will more easily follow the shape, contour and surface textures of the envelopes they are injected into and can be considered as “passive” with processing generally carried out at room temperatures and without the need for excessive mechanical pressing or vacuum extraction.

In some materials the polymeric chains are classified as fully crosslinked subsequently providing a viscoelastic behaviour that can demonstrate relatively low dependency when considered over temperature, time and under load duration.
LOCA materials are designed to manufacture laminated glass composites for use in most standard applications, used in both building product and automotive applications where primary requirements are safety and structural stability. Increasing focus is now placed on the use of LOCA products to embed and encapsulate passive and dynamic functions whilst providing mechanical stability and protection against potentially damaging environmental conditions.
Primary benefits of the materials and processing systems are identified as being:
- Easily flowing materials
- Cross linked material with a low temperature dependency and predictable behaviour when considering time over temperature
- Green lamination using little energy in processing and providing
- Passive curing profiles allowing safe lamination of materials with increased sensitivity to temperature and mechanical pressure
- High optical qualities
- Flexible wet chemistry to adapt material stiffness particularly useful when considering embedment for function in glass.
2. Chemistry
LOCA materials can be based on polyurethane or acrylate platforms and generally fall into the following package groups:
i) Polyurethane based catalytic curing
ii) Acrylic based cured with ultraviolet light
iii) Acrylic based catalytic curing materials
i) Polyurethane systems are generally designed with 2 parts formed by a base polyol material (component A) and corresponding isocyanate-based component (B component). The curing mechanism is often a catalytical poly-addition reaction with a function that may absorb small amounts of atmospheric moisture within the envelope and moisture captured on the surface of the substrate from the glass cleaning process. These materials can be considered as hydrophobic with low residual moisture contents. However excess moisture present during the curing phase should be avoided as it can create a side reaction within the isocyanate forming carbon dioxide that consequently forms a matrix with bubble inclusions.

Conditioning of both components requires de-gassing and adhesion is achieved via a reaction of the PU material with the glass surface and enhanced by adhesion promotors.
ii) Single part ultraviolet curing acrylic-based materials include an embedded photo-initiator that starts the radical polymerization under UVA exposure thus resulting in formed polymeric chains. The inclusion of co-monomers can enhance or tune properties such as tensile strength for impact performance.

iii) Multi component catalytic curing acrylic materials are often in 3 components based on a base material, catalyst and then a separate adhesion promotor. The curing mechanism is generally by radical polymerization via a catalyst thus the reaction only starts when the components are mixed.
Both curing mechanisms (the radical polymerisation of acrylic systems and the polyaddition of the PU systems) can be considered as exothermic.
Physics
Adhesion
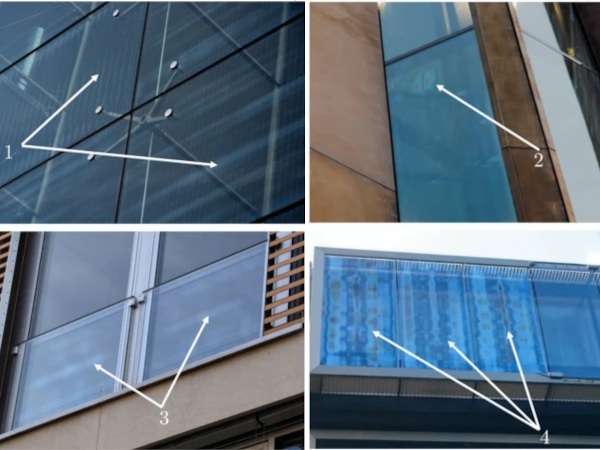
Example of the adhesion types (see example 1)
Substrate types
Lamination of most standard substrates is possible, however changes in type and thickness may require a change of interlayer thickness. Certain non-glass substrates require particular chemical preparations or pre-treatments.
Interlayer dimensions are key to effecting full cross linking thus offsetting the changing surface tension conditions and stiffness characteristics of differing glass types. As an example, interlayer thickness for float glass may increase from the standard 1.0mm up to 1.5mm and the maximum 2.0mm once the thickness of the float glass increases up to 6.0mm and then 8.0mm. Where the surface tensions of tempered glass increase and then the minimum interlayer thickness will automatically start at 1.5mm and then 2.0mm for all substrates thicker than a nominal design thickness of 5.0mm. This increase in interlayer thickness will also be required to accommodate for the changing roller wave and edge dip characteristics of heat-treated glass.
Plastics
In addition to glass substrates the bonding of glass to polycarbonate is enabled using specially developed PU based materials which have a naturalised chemical relationship to the surface of polycarbonate. The differential thermal expansion value of glass and polycarbonates, where the linear and / or volumetric expansion of polycarbonate is much greater than glass thus placing much greater tensions onto and into the interlayer material. Due to this differential expansion, it is important that the material bonding these two differing substrates combines a level of restraint or stiffness with creep and relaxation behaviour to compensate. Specially designed primers are used to enhance the adhesive properties of the glass surface to PUR.
i) Float glass
Processing is possible with all types of standard soda lime silicate float glass without the specific need to consider particular surface preparations for adhesion to the “tin” or “air” sides.
ii) Coated Glass
With adhesions to pyrolytic coated glass surfaces, consideration should be given to the interlocking adhesion of the metallised coatings to themselves in addition to the adhesion to substrate surfaces. The characteristics of these surfaces should always be considered before commencing preparation or processing.
3) Processing
i) Cleaning
Processing cleaning with the use of a standardised 3 stage warm water clean station, de-mineralised rinse and final air drying. Excessive residual moisture content resulting from the cleaning process and incidental humidity should be avoided if possible. Non-compliant incidental surface contaminations such as oils are normally treated with an isopropanol / de-ionised water mixture in a secondary cleaning process.
Any additional adhesion preparations e.g. primers will be applied to the glass surface at this point before finally rinsing with de-ionised water.
ii) Creation of the filling envelope
Creating a sealed enclosure uses a perimeter seal by way of one of the following methods:
a) Isobutylene material formed on a roll, which can be manually or automatically pressed at around 1 bar to provide the correct envelope depth. Butyl materials are considered as having thermal expansion values more aligned to those of the interlayer LOCA materials. They are also generally considered to provide superior barriers to moisture transmission however they are generally not transparent.

b) Preformed acrylic edge tapes at standard 1.0mm, 1.5mm and 1.8mm thickness. Full wetting of the adhesive faces can be improved using a primer and utilising a wetting out period of up to 24 hours ensuring that good adhesion is achieved. The recommendation to use tested, fully cured and approved materials will ensure that the:
- The product is fully cured and reduces the potential for uncured radicals migrating into the interlayer.
- There is a secondary moisture resistant barrier at any exposed edges.

c) Free formed edge sealing, free of a proprietary adhesive material, can be achieved by using specially designed mechanically applied systems.

iii) Mixing and metering of materials
With a temporary or permanent edge seal applied to the envelope the LOCA material can, where applicable, be mixed and subsequently metered into the envelope by way of an injection type process. Mixing is normally carried out at the point of delivery to avoid excessive purging post-application.

Calculation of the correct material volumes will be a function of the required interlayer thickness, area of the envelope and appropriate shrinkage values.
Shrinkage values will change for different materials ranging from 2% up to 13% by volume with the shrinkage values for polyurethane materials generally being lower than those of the acrylics.
PU materials require the efficient degassing of both A and B components before mixing and metering this can also be applied to single part acrylic materials where evacuation of the oxygen from the pre-cured material increases the efficiency of the polymerisation reaction.
Catalytic curing interlayers are mixed and metered in specially design dispensing units which area essential to manage the differing material densities to achieve the correct final mixed liquid density and the final density of the fully cured material.
Mixing ratios for both PU and acrylic catalytic curing materials will be calculated by weight and / or volume. The calculation of ratio values for 3-part acrylic materials will be different from that of PU based. Clearly single part ultra-violet curing acrylic LOCA materials do not require mixing.
Changes in ambient temperatures outside of those recommended for production 18 0 C – 23 0 C (dependant on the material type) can impact on the realisation of the correct mixing of the components. Controlling temperature in the production environment within the prescribed operating window is therefore important for achieving the optimum mixing of materials.
The pot life of different systems, although influenced by ambient temperatures can be adjusted for the catalytic systems.
iv) Curing
The method for curing the liquid material into the semi solid polymer changes dependant on the curing mechanisms as described above:
a) Single part acrylic-based materials are generally cured using UVA light radiated within a specific prescribed wavelength window. This activates the photo initiators embedded in the polymer, initiating the chemical reaction and forming the bonds and within the polymeric chains. UV light sources can be directed from above or both above and below.
Ambient temperatures outside of the recommended operating windows may prevent, retard or accelerate the reaction resulting in incomplete or accelerated curing resulting in localised stresses being formed. Differential curing can also be caused by a differential thickness across the interlayer that exceeds the allowed tolerance generally expressed as +/-0.5mm /m. This may result from the substrates being out of flatness or uneven curing tables. Tolerances should be set to guide the levels of acceptable uniform intensity for the sources of UV radiation. These can be either conventional blacklight tubes or alternatively UV LED units. Unequal radiation intensity outside of set parameters from the UV light sources must be controlled if excessive levels of differential stress are to be avoided.

Curing profiles can be monitored by measuring temperature changes resulting from the exothermic reaction. Completion of the reaction can generally be expected from 6 to 20 minutes. Changes in the types and thickness of substrates used will directly impact the intensities of UV radiation absorbed by the liquid interlayer and this changes the duration of the cure required. Additional time (safety factor) is generally added to the time taken to reach the maximum temperature to ensure that all materials are full and equally cured. This ensures that reliance is not placed entirely, only the area of the envelope that is monitored, thus reducing the opportunity for excessive differential curing.
b) With catalytic curing, materials curing is activated or accelerated by a chemical reaction between the catalyst component and the base material. Ambient temperatures will play an important part in the phasing of the reaction process. Measurement of the maximum temperatures and durations of the temperature cycle provides a quality management process for the effectiveness of the final cure.
Additional post cure checks can be carried out.
Glossary
- PUR / PU – Polyurethane resins
- Mma – Methylmethacrylate
- L.O.C.A – Liquid Optically Clear Adhesives
- T Max – Maximum measured temperature